Cellular power outage
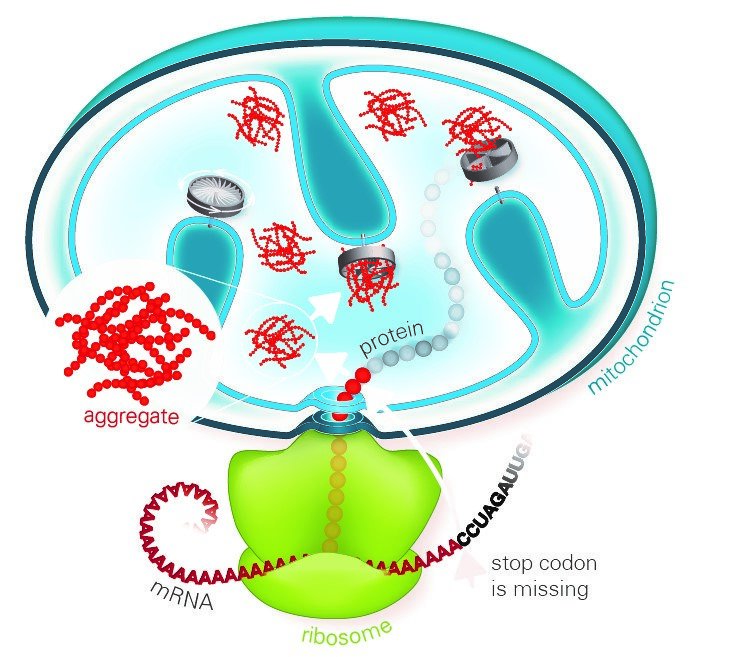
A common feature of neurodegenerative diseases such as Alzheimer's, Parkinson's or Huntington's disease are deposits of aggregated proteins in the patient's cells that cause damage to cellular functions. Scientists at the Max Planck Institute of Biochemistry and Ludwig-Maximilians-Universität in Munich report that, even in normal cells, aberrant aggregation-prone proteins are continually produced due to partial failure of the respiratory system. Unless they are removed by degradation, aggregates accumulate preferentially in the mitochondria, the cellular power plants, ultimately blocking energy production. In order to get rid of these toxic aggregates, cells have developed an elaborate protein quality control system.
Misfolded proteins made from defective blueprints are often sticky and clump together. Accumulation of such faulty proteins is known to contribute to the progression of several diseases. Therefore, cells have internal quality control mechanisms that detect and rapidly destroy faulty proteins. Proteins are produced by ribosomes, and misfolding can occur if they stall while decoding a damaged template. If the necessary ribosome-associated quality control machinery (RQC) does not function properly, defective proteins accumulate and form toxic aggregates in the cytoplasm of the cells. A previous study reported that this aggregation mechanism is mediated by so-called CAT-tails – C-terminal alanine-threonine sequences that are added to the defective proteins. So far, studies have focused on how the RQC recognizes and clears blocked ribosomes in the cytosol. The collaborating groups at the Max Planck Institute of Biochemistry and the university have now investigated the clearance of ribosome-blocked proteins destined for the mitochondria.
